Share
Pin
Tweet
Send
Share
Send

The penultimate stage on the way to obtaining a noble metal from radio components is the restoration of the latter from the corresponding chloride. Iron (II) sulfate is excellent for these purposes. Consider the equation of this reaction:

HAuCl4 - hydrogen tetrachloroaurate (III) - a compound that is formed when gold is dissolved in aqua regia. We will not delve into this process yet, since it deserves a separate article.
So, sulfate (hereinafter referred to as iron (II) sulfate) is one of the cheapest and readily available reducing agents. In stores it can be found under the name "iron sulfate", this is fertilizer. But this is not a pure substance, the proportion of sulfate in it is about 50%, the rest is impurities. And before restoring gold, it is necessary to purify our sulfate.
Will need
For this you need:
- Iron sulfate (sold in stores as "all for giving"; I bought a kilogram so that it was enough for my head);
- A solution of sulfuric acid, it is an acidic electrolyte for batteries (in car dealerships);
- Alcohol, I used 95%;
- Chemical or disposable tableware (glasses, stirring spoons).

We get iron sulfate from fertilizer
To prepare the solution, I use a glass jar. Inside 500 ml of hot water, you can slightly less.

Now measure out about half a glass of fertilizer:

As you can see, the substance is yellow-brown in color, you can use such a dirty "sulfate" unless for its intended purpose - spray the vegetation. We have other goals.
We fill it in a jar:

The solution acquired an unpleasant brown color. Staining occurred due to the reaction of fertilizer with water:

Iron in sulfate is oxidized to trivalent, and iron (III) hydroxide precipitates, which is actually the color due to it.
In order to obtain the sulfate of interest to us from the resulting compounds, it is necessary to acidify the solution with sulfuric acid. Add in small portions until the solution brightens.

The following reactions occur:

Hydroxide interacts with acid, and iron (III) sulfate is formed (ion exchange reaction). It, in turn, reacts with atomic hydrogen, which is formed during the dissociation of sulfuric acid. The latter reaction is redox.
Now the solution contains exactly the sulfate we need, but the solution itself is still cloudy. We let it stand and filter it, I used a chemical filter.


Insoluble impurities remained at the bottom of the can:

We rinse it and pour the filtered solution there. It is much brighter and cleaner than the original.

Ethyl alcohol displaces salts of dibasic acids from their solutions. We will use this property to obtain solid sulfate. Pour alcohol into the solution, I poured 200ml, which is equal to slightly more than half of the initial fertilizer volume.
Crystals of our sulfate appeared at the bottom.

I left the solution for two days so that all the sulfate had time to precipitate.

You can initially pour more alcohol to make this process faster.
We drain the liquid, by tapping on the bottom and gentle heating, we separate the sulfate from the can and pour it on a paper towel to dry.

After a few hours, pour the sulfate into a glass jar, sign the substance, and in this form it can be stored for a long time.

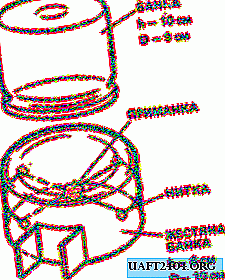
Since crystalline hydrates precipitated, the formula of the substance is as in the picture. But this does not stop us from using it for our purposes, dissolving in water anyway.
Conclusion
Safety and compliance with acid handling are a matter of course. In no case do not forget about them.
All pure substances!
Share
Pin
Tweet
Send
Share
Send