I was just bewitched when I repeated this beautiful and exciting experiment! It does not contain hard-to-reach reagents, all the components are in the kitchen - it is baking soda and vinegar.
This experiment is called "hot ice." It can be repeated by anyone!
We will need:
- - 1 kilogram of vinegar 70%.
- - 978 grams of baking soda.
- - 200 milliliters of water.
Mix all the ingredients in a plastic container. And we observe how the chemical reaction of soda with a bite occurs with the release of carbon dioxide. When mixing, be careful with acetic acid, the thing is still dangerous and requires special attention.
After a few hours, we received sodium acetate trihydrate. Transfer this white substance into an enamel pan and start heating. We need to melt the sodium acetate trihydrate in a saucepan. After a while we get a clear liquid.
Place the solution in a bucket of cold water and cover with a lid. After cooling, our hot ice will be ready.
From the previous capacity, we should have left a few grams of sodium acetate trihydrate in crystalline form, before heat treatment.
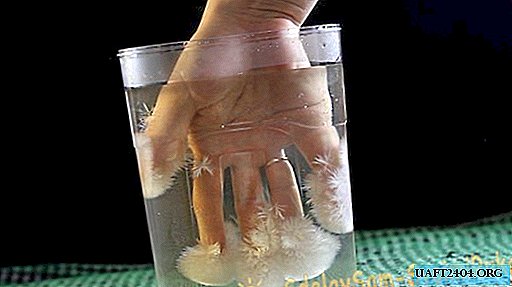
So, even a small crystal of sodium acetate is able to start the crystallization process in a chilled solution.
If you now immerse your hand in the solution and there are crystals of sodium acetate at your fingertips, then the entire solution will crystallize quickly.
Repeat this wonderful experiment, and be sure to send the photo in the comments, everyone will be interested to see your result.